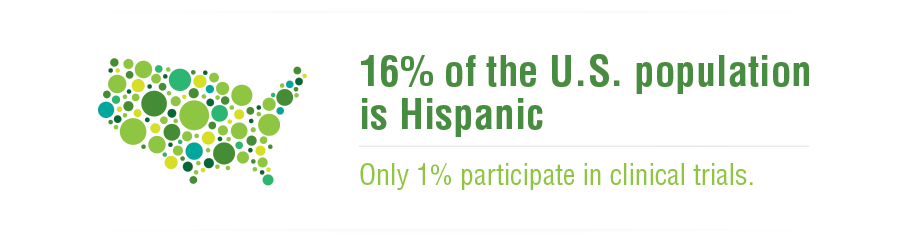
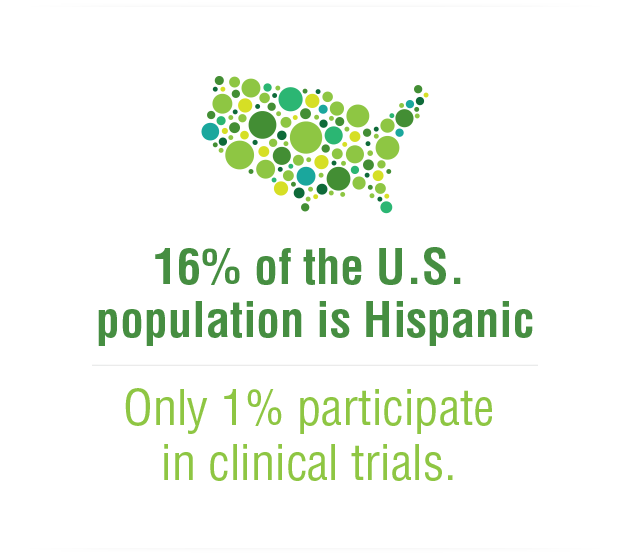
Every time you take a medicine – even if it’s just for a headache – you are benefitting from the results of a clinical trial. By the time a medicine reaches the pharmacy counter, it has withstood rigorous testing to ensure safety and efficacy.
The clinical trials process is lengthy and complex, relying heavily on volunteer participation, and without these volunteers, the development of new medicines would not be possible.